As recently as 2014 and 2015, pharmaceutical publications were lamenting the lack of reliable, non-destructive in-line testing methods for Fill and Finish packaging processes relative to glass vials. The advent of in-line testing is changing how pharmaceutical companies can optimize packaging processes and container designs; enabled by an improved understanding of mechanical loads like impact, pressure and vertical force.
Optimization of packaging processes is top of mind for leaders in pharmaceutical manufacturing. At the upcoming Healthcare+ Packaging Expo, the education series is focused on topics like ‘Improving Asset Utilization in Pharmaceutical Packaging’ and ‘The IIoT Promise: Improving OEE and Machine Reliability’.
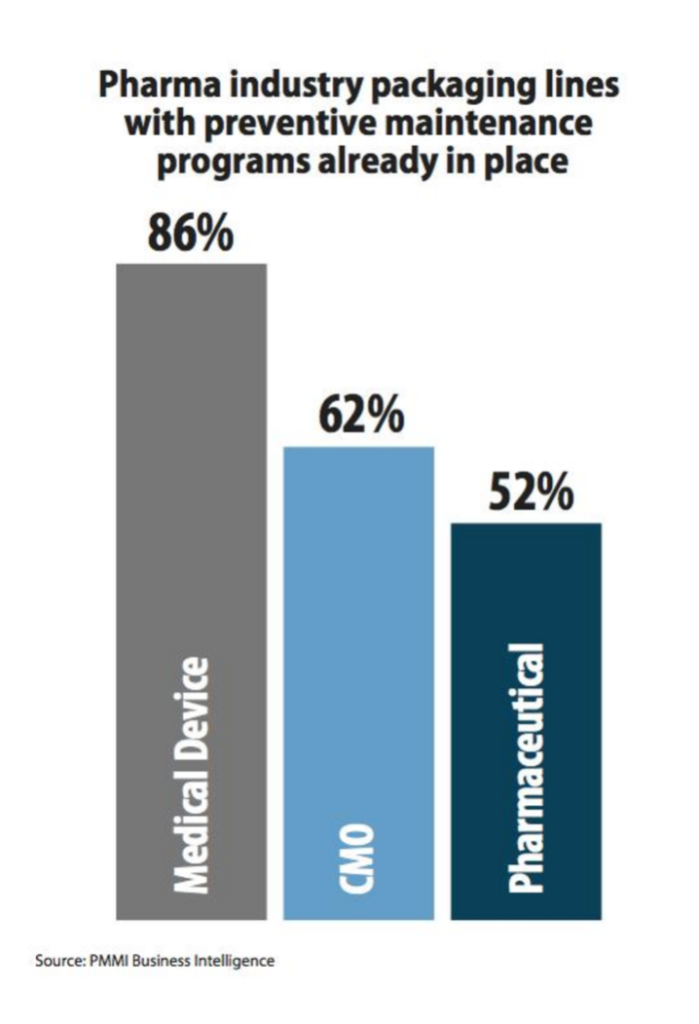
Preventative maintenance is also a hot topic with one pharma VP of manufacturing commenting, “There will be massive growth in preventive maintenance to improve uptime,” in the latest study from PMMI Business Intelligence.
The same study reports that respondents are seeking improvements relative to “better access to the machine, life cycle reporting on parts, universal part replacement, improved data collection on machine efficiency, and clear instructions on HMI tied into status bar with dashboard display.”
New data can optimize efficiency, as well as informing preventative maintenance and capital investments.
The old adage is that you ‘can’t optimize what you can’t measure’ and the fact is, there are many invisible variables in any given packaging process that are counter-productive to optimization efforts.
Mechanical loads including pressure, impact and vertical force are such variables. A primary source of surface flaws, glass breakage (and related downtime) and even equipment degradation over time, excess mechanical force can cause a number of inefficiencies:
• Unnecessary waste and container damage
• Unplanned downtime
• Inefficient equipment integrations
• Lack of data informing maintenance prioritization
• Risks associated with glass breakage and/or product contamination
“Until the advent of in-line sensor technology, impact measurement was a static measurement. It was not possible to understand the root cause of shock in production, nor was it possible to visualize impact on the line in real-time. This is key to enabling fillers to improve their line efficiency and reduce damage to their glass containers,” explains Pablo Asiron, Executive VP of Global Sales for Masitek, the leading supplier of smart in-line sensor technology.
A non-destructive testing method, smart sensors are fitted into an equivalent replica of a glass vial or container. The smart in-line sensor measures variables during processing in a manner that’s directly equivalent to how containers being processed experience force.
Precise information regarding mechanical load, like the impact threshold a glass vial would face during processing, enables packaging engineers to quantitatively assess the right course of action to correct inefficiencies.
With this information, users can:
• Pinpoint the escalating areas of issue prior to a production run to minimize downtime.
• Find the exact location of damage causing impacts and pressure in minutes to avoid lengthy shut-downs.
• Expedite changeovers with data-driven calibration
• Identify equipment degradation over time to target maintenance and prioritize capital investments
• Support quality and continuous improvement initiatives
Understanding mechanical force to improve container design and support sustainable packaging initiatives.
As is true with consumer brands, pharmaceutical brand leaders are also under increased pressure to maximize the use of recycled or renewable materials and establish green packaging designs. For an industry that has not traditionally focused R and D on packaging innovation, sustainability, including development or modification of a package to reduce its environmental impact, is a relatively new concept.

With a growing focus on sustainable products, there is an emphasis on environmentally friendly designs and materials used for pharmaceutical packaging. Packaging sourced from more sustainable materials, and less of it in general, is the goal. However, for the pharmaceutical industry, this challenge is even greater as the industry is subject to government safety regulations, patient compliance, unit-dose control, combating counterfeits, and providing child-resistant packaging.
Source: Contract Pharma, Pharmaceutical Packaging Market Trends, 2019.
Many pharmaceutical packaging operations are forming sustainability teams to address these initiatives. To succeed, teams need to deploy an approach that includes sustainable or lightweight container designs, efficiency in processes, optimized equipment and streamlined transportation; all without compromising patient safety.
Given the complexity of pharmaceutical packaging, the accurate measurement of mechanical force in real-world scenarios can be an invaluable tool to capture the nuances of designing for sustainability, be it equipment optimization or testing innovative container designs:
• Assist in testing new product designs prior to production
• Test and optimize new packaging, including closures, lightweight containers, etc.
• Establish thresholds for all lines and products to ensure packaging design and materials won’t damage in production. Using these thresholds across the organization can assist in expediting new product packaging to market
• Optimize transportation processes
Incorporating mechanical load as a data source at the beginning of product development creates new opportunities to develop solutions that meet efficiency targets, regulatory requirements and sustainability targets. This information can help determine where changes can be made to make packaging more machine-ready or how packaging can be adapted without compromising patient protection.
In an increasingly competitive environment, pharmaceutical packaging teams need every available advantage.






